At present, the synthesis technology of polypeptide is very mature, but DG rearrangement or aspartate amidation sometimes occur in the process of polypeptide synthesis, resulting in the impurity peak close to the target peak, which is difficult to purify or even can not be separated.As a result, the yield is reduced. To solve such problems, a side-chain protective group of Asp, cyanothionyl, or CYS, is introduced today.Such methods can effectively reduce the impurities caused by DG rearrangement and Asp amidation, and greatly improve the synthesis and purification efficiency.Later on similar side reactions, such as DG rearrangement and Asp amidation, are collectively referred to as D amidation.
One and two are commonly used strategies at present, and three are protection-based strategies recommended today.
一. Side chain tert-butoxy
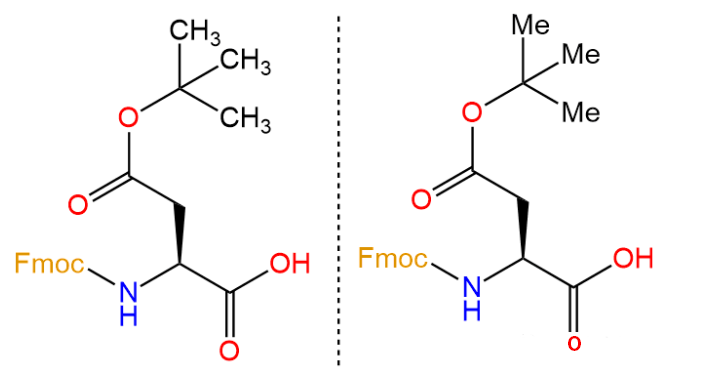
Abbreviated as OtBu, amino acid abbreviation Asp (OtBu)
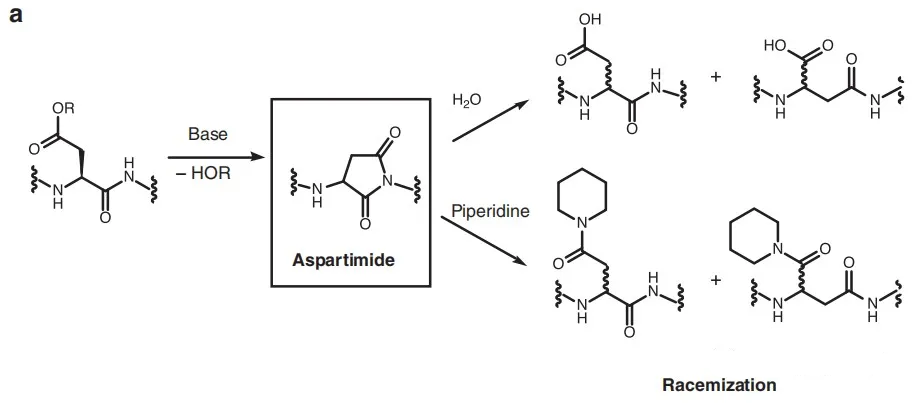
In some special sequences, such methods are prone to racemization and impurity generation, as shown in the following figure
二. Dipeptide structural unit
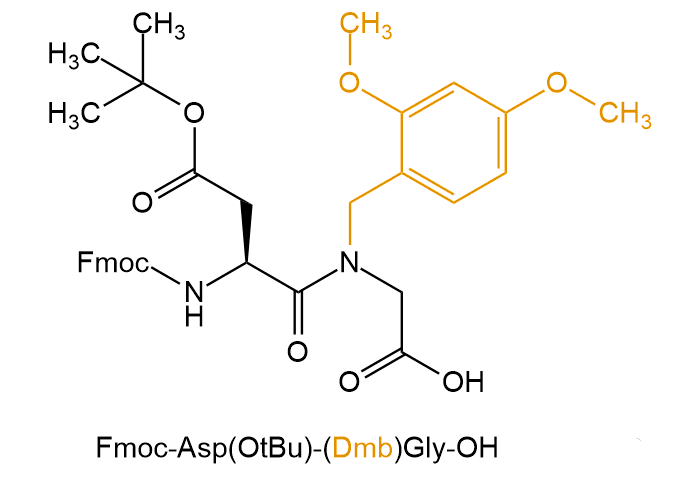
For example, use Fmoc-Asp (OtBu) -(Dmb) Gly-OH and Fmoc-Asp (OtBu) -(Hmb) Gly-OH
Dmb:Dimethoxybenzyl
Hmb: 2-hydroxy-4-methoxy-benzyl
Such schemes do reduce the chances of D amidation (usually with the addition of HOBT or other additives to further reduce the chances of side reactions), but the coupling efficiency is very low and the dipeptide must be used as the unit structure for coupling.And it can not prevent the side reaction of D amidation very effectively.
三、 Cyanothionyl (CYS)
1, Formation of Cyanothionyl Groups
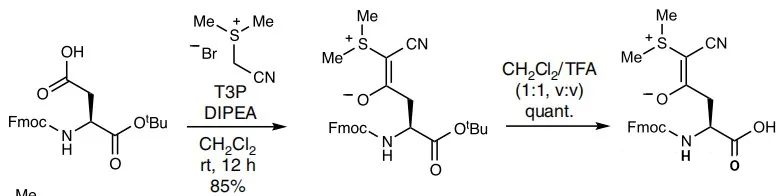
2, Removal of Cyanothionyl
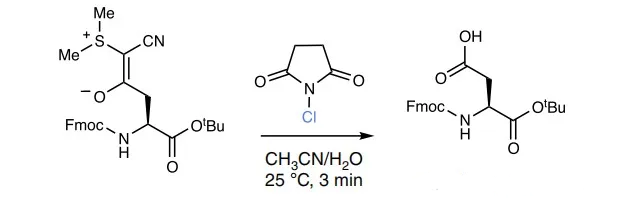
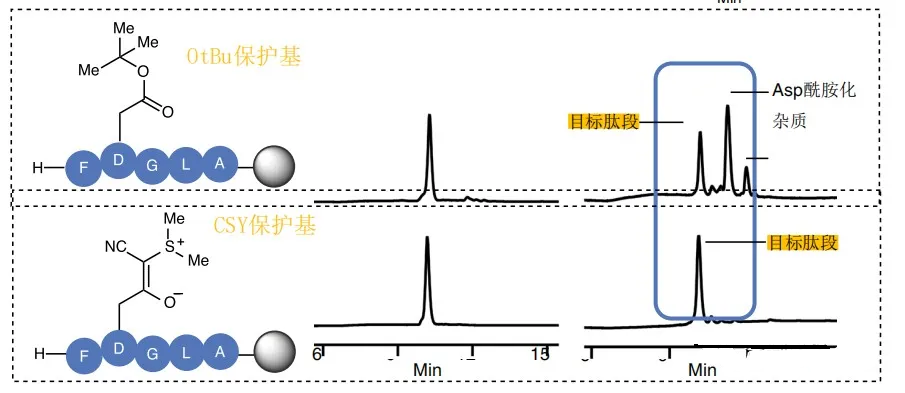
3, Effect contrast display
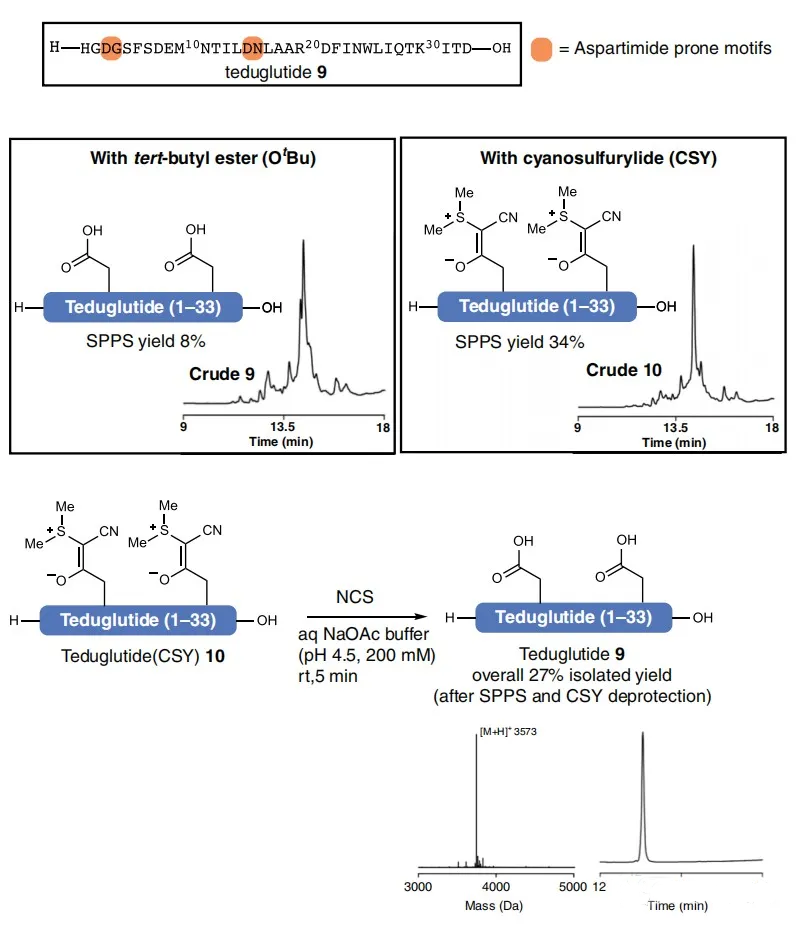
4, Example Display (Application of CSY Protective Groups to the Synthesis of Tidulutide)